Background: Primary central nervous system lymphoma (PCNSL) is an aggressive extranodal subtype of non-Hodgkin's lymphoma (NHL). High-dose methotrexate (HD-MTX)-based regimens are the first-line treatment for PCNSL, but remain suboptimal due to response instability and short effective remission duration. Orelabrutinib is a novel, potent, highly selective BTK inhibitor with a high CSF concentration. This study aims to investigate optimal combination dose of Orelabrutinib in combination with Rituximab, Methotrexate, and Dexamethasone (ORMD) as well as safety and efficacy of the regimen.
Methods: This was a phase 1, investigator-initiated, dose-escalation study of ORMD, being conducted at Huashan Hospital, Fudan University (NCT05036577). Eligible patients must have histopathologically confirmed PCNSL-DLBCL by biopsy of brain lesions and be aged 18-75 years with adequate organ functions. Patients were treated for 6-8 cycles of induction therapy with 21 days per cycle, receiving rituximab (375mg/m 2 on day 1), dexamethasone (10-15mg on d1-d4), MTX (d2, 3.5g/m 2 or 5g/m 2), and orelabrutinib (once daily, after MTX clearance, 150mg/d, or 200mg/d), followed by orelabrutinib maintenance up to one year among CR/CRu patients. The primary objective was to determine the maximum tolerated dose (MTD) of the combination of orelabrutinib and MTX with R and D and investigate the safety and tolerability of this regimen using Bayesian Optimal Interval (BOIN) waterfall design to determine rule of dose escalation and movement among dose combination matrix to identify MTD contour. DLT was defined by the occurrence of severe toxicities during the first cycle: any grade 4 hematologic toxicity, grade 3 febrile neutropenia and grade 3 thrombocytopenia with hemorrhage, or any grade 3 non-hematologic toxicity that failed to respond to supportive therapy and possibly related to orelabrutinib and/or MTX (assessed according to NCI CTCAE V5.0). The secondary objectives included ORR/CR(u) and PFS/OS.
Results:
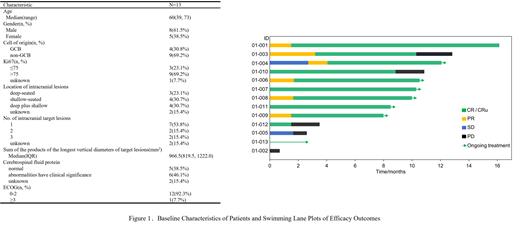
From October 2021 to July 2023, the study completed enrollment of all 13 patients. The median age was 60 years, and other basic characteristics are shown in Figure 1. All the patients had completed at least one cycle of ORMD treatment and were evaluable for DLT. The median cycle of ORMD was seven (range: 2.5-8).
No DLT occurred among first 3 patients in cohort 1 (Orelabrutinib: 150mg/MTX: 3.5g/m 2) and dose escalation was allowed to cohort 2 (Orelabrutinib: 200mg/MTX: 3.5g/m 2). In cohort 2, 5 patients were dosed in total, and Gr 3 lung infection was observed in patient #4 during cycle 1, was deemed as a DLT. Another four patients were enrolled in the same cohort according to BOIN design algorithm and no DLT was identified, allowing to escalate to cohort 3 (Orelabrutinib:200mg/MTX:5g/m 2). In cohort 3, 5 patients were dosed in total, and Gr 3 elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was observed in patient #9 during cycle 1, was deemed as a DLT. The causality of this AE was certain to methotrexate due to folate metabolic gene polymorphism in the assessment of the investigator. The toxicity probability from 3 dose combo cohort is 0.13, 0.13 and 0.21, all of which is below predefined threshold 0.3. So MTD is not reached and 200mg+5g/m 2 dose combo remains to be RP2D candidate. No Gr 4 AE had been observed. The most common adverse events were lymphopenia (Gr 3 in 8 case), neutropenia(Gr 3 in 2 case), Covid-19(Gr 3 in 2 case) and hyperglycemia (Gr 3 in 2 case). No atrial fibrillation or bleeding was observed by the cut-off date.
ORR and CR/CRu in 12 patients possessed at least one efficacy evaluation were both 83.3%, the PFS and OS rate were 56.6% (95% CI, 29.6% ,100%) and 100%(95% CI, 100% ,100%) at 1 year. Among nine patients completed ≥6 cycles of induction therapy, ORR and CR/CRu were both 100%. PFS and OS rate were 75.0% (95% CI, 42.6% ,100%) and 100%(95% CI, 100% ,100%) at 1 year. All patients in remission achieved CR/CRu, the high rate of deep remission may be related to the addition of orelabrutinib.
Conclusions: As a novel therapeutic combination of induction therapy, ORMD therapy has shown deep remission potential as well as a predictable and manageable safety profile in patients with newly diagnosed PCNSL. MTD contour not reached. The combination of this induction therapy with consolidation therapies such as ASCT deserves further investigation and may provide greater survival benefit for patients.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Orelabrutinib is a novel selective Bruton Tyrosine kinase(BTK)inhibitor. BTK inhibitors are highly effective in B cell NHL which carried MYD88 and/or CD79b mutations. PCNSL has high frequency of MYD88/CD79b mutations.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal